Subscribe to our Telegram channel to get a daily dose of business and lifestyle news from NHA – News Hub Asia!
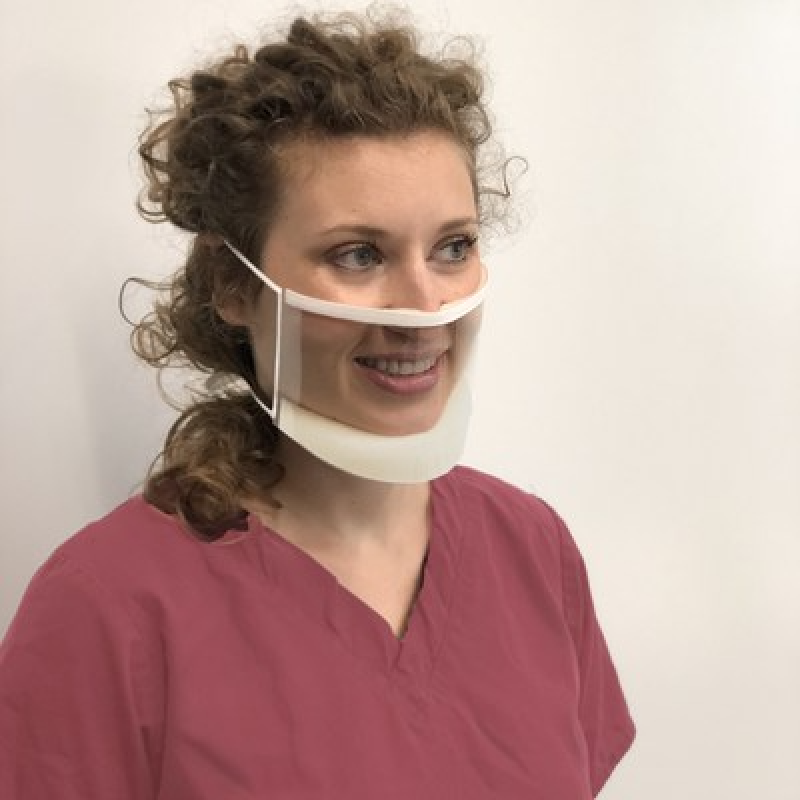
The ClearMask™ transparent surgical masks meet the CE Marking requirements as a Class I medical device under the European Union Medical Device Regulation (2017/745) and can be used in hospitals, clinics, schools, and other settings. The mask is optimized for maximum clarity and comfort, meeting applicable EN 14683 requirements for fluid resistance, microbial cleanliness, and biocompatibility, which offers a high level of protection for medical use in environments such as operating rooms.
In the United States, the ClearMask™ brand received FDA clearance as a Class II medical device on April 6, 2020, and meets applicable ASTM Level 3 requirements in the United States for fluid resistance and flammability. The ClearMask™ transparent surgical mask also passed additional tests in accordance with the transparent mask guidelines released by the United Kingdom, including tests for resistance to penetration of microorganisms (ASTM F1671) and visibility (EN 166).
During the pandemic, the ClearMask™ brand has helped provide protection against the transfer of aerosols, fluids, and sprays, and has been widely adopted as the new standard to help improve communication and trust where masks are required. With its fully transparent, anti-fog plastic barrier, the ClearMask™ brand increases visual communication, which may help avoid costly errors and adverse outcomes. As demonstrated in a first-of-its-kind study in the Journal of the American Medical Association, the ClearMask™ is also critical in establishing rapport and earning trust.
The company started developing the transparent mask in 2017 after their deaf co-founder suffered an adverse experience during her surgery. Traditional surgical masks blocked her providers’ faces, impeding effective communication and essential safety checks.
“When the pandemic began, our mission was to bring a human-centered mask to everyone who needed it, especially children, older adults, deaf and hard of hearing people, and those who heavily depend on visual communication. ClearMask continues to fight against the current pandemic and we are here to stay,” said Allysa Dittmar, President of ClearMask.
To date, the company has provided over 18 million masks in the United States and globally. The company has partnered with several distributors, including Cardinal Health, Grainger, Henry Schein, McKesson, and Oaktree Products, and is currently onboarding distributors for the CE-marked masks. The masks can be purchased in the United States through ClearMask’s website at www.buy.theclearmask.com, and countries outside the United States may purchase the masks here.
Source: ClearMask(Press Release)